Globular proteins can undergo a liquid-liquid phase separation, termed complex coacervation, with oppositely charged polyelectrolytes. This macromolecule rich liquid phase can be used to stabilize the protein component, mimic the cytoplasmic environment, or enhance protein activity. However, due to the relatively low charge density and “patchy” charged surface of most proteins, many globular proteins do not phase separate with oppositely charged polymers. Genetic modification of proteins enables the systematic study of protein parameters that influence complex coacervation. We also seek to engineer this liquid-liquid phase separation behavior into proteins of interest for applications in drug delivery, protein purification, and protein stabilization.
Learn more about our Work on complex coacervation:
Cummings, C.S.; Obermeyer, A.C.* Phase separation behavior of supercharged proteins and polyelectrolytes. Biochemistry, 2018, 314. [link]
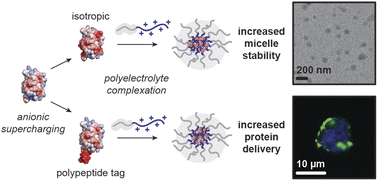
Kapelner, R.A.; Obermeyer, A.C.* Ionic polypeptide tags for protein phase separation. Chemical Science, 2019, 10, 2700. [link]
Horn, J.M.; Kapelner, R.A.; Obermeyer, A.C.* Macro- and Microphase Separated Protein-Polyelectrolyte Complexes: Design Parameters and Current Progress. Polymers, 2019, 11, 578. [link]
Zervoudis, N.A.; Obermeyer A.C.* The effects of protein charge patterning on complex coacervation. Soft Matter, 2021, 17, 6637-6645. [link]
Kapelner, R.A.; Fisher, R.S.; Elbaum-Garfinkle, S.; Obermeyer, A.C.* Protein charge parameters that influence stability and cellular internalization of polyelectrolyte complex micelles. Chemical Science, 2022, 13, 14346. [link]
Horn, J.M.; Zhu, Y.; Ahn, S.Y.; Obermeyer, A.C.* Self-assembly of globular proteins with intrinsically disordered protein polyelectrolytes and block copolymers. Soft Matter, 2022, 18, 5759-5769. [link]
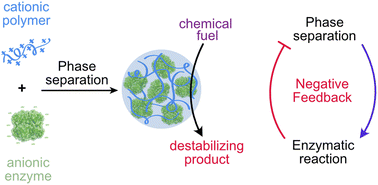
Modi, N.; Chen, S.; Adjei, I.N.A.; Franco, B.L.; Bishop, K.J.M.*; Obermeyer, A.C.* Designing negative feedback loops in enzymatic coacervate droplets. Chemical Science, 2023, 14, 4735-4744. [link]
Additional context on Complex Coacervation
Complex Coacervation: Principles and Applications
(special issue in Advances in Colloids & Interface Science)
Mills, C.E.; Obermeyer, A.C.; Dong, X-H.; Walker, J.; Olsen, B.D. Complex Coacervate Core Micelles for the Dispersion and Stabilization of Organophosphate Hydrolase in Organic Solvents. Langmuir, 2016, 32, 13367-13376. [link]
Obermeyer, A.C.; Mills, C.E.; Dong, X-H.; Flores, R.J.; Olsen, B.D. Complex coacervation of supercharged proteins with polyelectrolytes. Soft Matter, 2016, 12, 3570-3581. [link]